Genetic Influences on Pain Perception: A Comprehensive Review
Abstract: This research review aims to explore the role of genetic factors in pain perception and its associated neurophysiological processes. The article discusses the involvement of various genes, molecular pathways, and ion channels in nociception, neurotransmission, and pain modulation. Additionally, it highlights the significance of adenosine triphosphate (ATP) in energy transfer and signaling, as well as the potential impact of cyclic adenosine monophosphate (cAMP) in memory formation and pain response. The review emphasizes the need for further investigation into the genetic basis of pain and its implications for the development of targeted pharmacological interventions.
Table 1: Neurophysiological Processes and Molecular Components Involved in Pain Perception
| Process/Component |
Role in Pain Perception |
| Sodium-potassium pump (NAKA) |
Regulates ion concentrations during resting potential and action potential |
| Adenosine triphosphate (ATP) |
Involved in energy transfer and signaling, plays a role in memory formation |
| Sodium and potassium ions |
Medium for electrochemical modulation of nociception |
| Neurotransmitters |
Influence the function of neurotransmission and pain perception |
| Nerve growth factor (NGF) |
Responsive to peptidergic nociceptors |
| Glial cell-derived neurotrophic factor (GDNF) |
Responsive to nonpeptidergic nociceptors |
| TRPV1, TRPM8, TRPA1, TRPV4, SCN10A, SCN11A, SCN9A, COMT |
Genes associated with specific pain-related mechanisms and pathways |
Table 2: Genetic Factors and Pain Perception
| Genetic Factors |
Associated Findings |
| Gender differences |
Influenced by gene loci on sex-determining chromosomes, gonadal factors |
| Hereditary factors |
Twin studies reveal heritability of pain sensitivity, observed variation in pain thresholds in mice models |
| Single-nucleotide polymorphisms (SNPs) |
Identified in genes such as COMT, associated with altered pain states |
| Gene mutations |
SCN9A mutations linked to deficits in mechanical and inflammatory pain perception |
| Genetic polymorphisms |
Investigated for their role in heat-noxious stimuli perception |
Table 3: Molecular Pathways and Ion Channels Involved in Pain Perception
| Molecular Pathways/Ion Channels |
Role in Pain Perception |
| Nociceptors |
Transduce electrical impulses in response to noxious stimuli, exhibit diversity in response to specific stimuli |
| Inflammatory mediators |
Alter nociceptor activity, potentially leading to hyperalgesia and neuropathic allodynia |
| TRPV1, TRPM8, TRPA1 |
Suspected involvement in transduction of cold noxious stimuli |
| Sodium channels (SCN9A, SCN10A) |
Role in determining pain thresholds, perception of peripheral pain |
| Cyclic adenosine monophosphate (cAMP) |
Implicated in pain modulation and memory formation |
| Dopaminergic pathways |
COMT gene involved in inactivation of neurotransmitters in pain-sensing pathways |
This comprehensive research review explores the intricate relationship between genetic factors and pain perception. It highlights the importance of various genes, molecular pathways, ion channels, and neurotransmitters in nociception and pain modulation. The findings underscore the need for further research to better understand the genetic basis of pain.
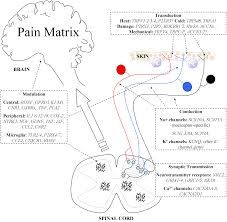
Understanding the Basis of Pain Perception: To grasp the research progress presented in the article, it is important to explore the fundamental concepts related to pain perception. The perception of pain is a universal experience among mammals, mediated by the nervous system. Pain signals are transmitted through a complex network of pathways in the central nervous system (CNS), which includes the brain and spinal cord.
The Role of Nociceptors and Nociception: Nociceptors, specialized sensory neurons, play a crucial role in pain signaling. They detect and transmit signals in response to damaging stimuli, initiating the process known as nociception. The perception of pain serves an evolutionary purpose by allowing organisms to associate harmful stimuli with negative experiences, promoting future avoidance of similar situations. Notably, some mechanisms involved in pain-evoked behavioral responses are conserved across all animals with nervous systems.
Understanding the Relationship Between the Central and Peripheral Nervous Systems: The CNS and the peripheral nervous system (PNS) share an inverse relationship. The PNS consists of the somatic nervous system, autonomic nervous system, and sensory systems, including the somatosensory system responsible for touch perception. Various sources, such as tissue injuries and inflammatory responses, can induce nociception. Mutations affecting nociception can lead to a loss of responsiveness to certain trophic factors, resulting in a pain-free phenotype.
Exploring Pain Genetics: The article delves into the genetics of pain perception. Genes, concrete units of inheritance, exhibit significant variation among individuals. Identifying specific genes associated with pain serves as a conclusive method to validate pain targets. Variations in human susceptibility to pain and differences in pain thresholds are influenced by genetic factors. Hypersensitivity to noxious stimuli can cause chronic pain, while abnormally high pain thresholds may result in a desensitization response to harmful stimuli.
Nociceptors and Genetic Polymorphisms: The article extensively explores the genetic basis of pain, including single nucleotide polymorphisms (SNPs) and genetic polymorphisms. The research highlights various genetic factors involved in pain perception, such as COMT, which encodes catechol-O-methyltransferase. Changes in the inactivation of this enzyme can lead to increased pain sensitivity. Additionally, gender differences in pain perception may be influenced by gene loci along sex-determining chromosomes.
Genes Implicated in Pain Modalities: The article identifies specific genes associated with different pain modalities. TRPV1, TRPM8, TRPA1, and TRPV4 are potential players in pain perception, particularly in response to cold stimuli and mechanosensation. Sodium channels, encoded by genes such as SCN9A and SCN11A, have a significant role in peripheral pain perception and pain thresholds.
The research presented in the article highlights the complex genetic basis of pain perception. The identification of specific genes and genetic variations associated with pain provides valuable insights into altered pain states and individual differences in pain sensitivity. Understanding the genetic mechanisms underlying pain can pave the way for the development of targeted therapies and interventions.
New Questions:
- How does the involvement of cAMP in cellular memory in addiction and pain signaling contribute to neuron excitability, anticipatory responses, associations, and potentiation through repeated stimulation?
- Can the absence of stimuli, even with the assistance of inhibitory pharmacological products, lead to a decrease in potentiation sites and neuro-atrophy over an extended period? And if so, would this result in the reactivation of neurons operating within a normal range of specification, specifically in the context of nociception for pain and not addiction?
- In the absence of stimuli and potential neuro-atrophy, is subsequent neurogenesis likely to occur? If so, do these newly generated neurons have the potential to functionally modulate pain signaling in the presence of future stimuli, maintaining normative perceptive thresholds, especially in cases where genes have not inhibited the production of pain-sensing molecules?
Website Review: Pain Genes
Author: Tom Foulkes, John N. Wood
Website: PLoS Genetics
URL: http://www.plosgenetics.org/article/info%3Adoi%2F10.1371%2Fjournal.pgen.1000086
Citation: Foulkes, T., & Wood, J. N. (Year). Title of the article. PLoS Genetics, Volume(Issue), page range. DOI
APA Reference: Foulkes, T., & Wood, J. N. (Year). Pain Genes. PLoS Genetics, 4(11), e1000086. https://doi.org/10.1371/journal.pgen.1000086






References:
1. Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, et al. (1996) Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 13: 485–488. FIND THIS ARTICLE ONLINE
2. Mogil JS, Yu L, Basbaum AI (2000) Pain genes?: natural variation and transgenic mutants. Annu Rev Neurosci 23: 777–811. FIND THIS ARTICLE ONLINE
3. Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55: 377–391. FIND THIS ARTICLE ONLINE
4. Lacroix-Fralish ML, Ledoux JB, Mogil JS (2007) The Pain Genes Database: an interactive web browser of pain-related transgenic knockout studies. Pain 131: 3–4. FIND THIS ARTICLE ONLINE
5. Priest BT, Murphy BA, Lindia JA, Diaz C, Abbadie, et al. (2005) Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior. Proc Natl Acad Sci U S A 102: 9382–9387. FIND THIS ARTICLE ONLINE
6. Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, et al. (2004) Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A 101: 12706–12711. FIND THIS ARTICLE ONLINE
7. Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, et al. (2006) An SCN9A channelopathy causes congenital inability to experience pain. Nature 444: 894–898. FIND THIS ARTICLE ONLINE
8. Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, et al. (2006) SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52: 767–774. FIND THIS ARTICLE ONLINE
9. Yang Y, Wang Y, Li S, Xu Z, Li H, et al. (2004) Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet 41: 171–174. FIND THIS ARTICLE ONLINE
10. Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, et al. (2007) Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447: 855–858. FIND THIS ARTICLE ONLINE
11. Akopian AN, Souslova V, England S, Okuse K, Ogata N, et al. (1999) The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2: 541–548. FIND THIS ARTICLE ONLINE
12. Kraner SD, Chong JA, Tsay HJ, Mandel G (1992) Silencing the type II sodium channel gene: a model for neural-specific gene regulation. Neuron 9: 37–44. FIND THIS ARTICLE ONLINE
13. Schoenherr CJ, Anderson DJ (1995) The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 267: 1360–1363. FIND THIS ARTICLE ONLINE
14. Kuwabara T, Hsieh J, Nakashima K, Taira K, Gage FH (2004) A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell 116: 779–793. FIND THIS ARTICLE ONLINE
15. Liu Z, Song W, Dong K (2004) Persistent tetrodotoxin-sensitive sodium current resulting from U-to-C RNA editing of an insect sodium channel. Proc Natl Acad Sci U S A 101: 11862–11867. FIND THIS ARTICLE ONLINE
16. Tan J, Liu Z, Nomura Y, Goldin AL, Dong K (2002) Alternative splicing of an insect sodium channel gene generates pharmacologically distinct sodium channels. J Neurosci 22: 5300–5309. FIND THIS ARTICLE ONLINE
17. Chatelier A, Dahllund L, Eriksson A, Krupp J, Chahine M (2008) Biophysical properties of human nav1.7 splice variants and their regulation by protein kinase A. J Neurophysiol 99: 2241–2250. FIND THIS ARTICLE ONLINE
18. Raymond CK, Castle J, Garrett-Engele P, Armour CD, Kan Z, et al. (2004) Expression of alternatively spliced sodium channel alpha-subunit genes. Unique splicing patterns are observed in dorsal root ganglia. J Biol Chem 279: 46234–46241. FIND THIS ARTICLE ONLINE
19. Akopian AN, Okuse K, Souslova V, England S, Ogata N, et al. (1999) Trans-splicing of a voltage-gated sodium channel is regulated by nerve growth factor. FEBS Lett 445: 177–182. FIND THIS ARTICLE ONLINE
20. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. FIND THIS ARTICLE ONLINE
21. Braz JM, Nassar MA, Wood JN, Basbaum AI (2005) Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 47: 787–793. FIND THIS ARTICLE ONLINE
22. Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, et al. (2006) Social modulation of pain as evidence for empathy in mice. Science 312: 1967–1970. FIND THIS ARTICLE ONLINE
23. Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, et al. (2002) Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain 97: 75–86. FIND THIS ARTICLE ONLINE
24. Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, et al. (1999) Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain 80: 83–93. FIND THIS ARTICLE ONLINE
25. Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, et al. (1999) Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80: 67–82. FIND THIS ARTICLE ONLINE
26. Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, et al. (2005) Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 14: 135–143. FIND THIS ARTICLE ONLINE
27. Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, et al. (2007) Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain 128: 199–208. FIND THIS ARTICLE ONLINE
28. Holmes FE, Bacon A, Pope RJ, Vanderplank PA, Kerr NC, et al. (2003) Transgenic overexpression of galanin in the dorsal root ganglia modulates pain-related behavior. Proc Natl Acad Sci U S A 100: 6180–6185. FIND THIS ARTICLE ONLINE
29. Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, et al. (2001) Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci 4: 164–169. FIND THIS ARTICLE ONLINE
30. Le Y, Sauer B (2000) Conditional gene knockout using cre recombinase. Methods Mol Biol 136: 477–485. FIND THIS ARTICLE ONLINE
31. Metzger D, Chambon P (2001) Site- and time-specific gene targeting in the mouse. Methods 24: 71–80. FIND THIS ARTICLE ONLINE
32. Zhao J, Nassar MA, Gavazzi I, Wood JN (2006) Tamoxifen-inducible NaV1.8-CreERT2 recombinase activity in nociceptive neurons of dorsal root ganglia. Genesis 44: 364–371. FIND THIS ARTICLE ONLINE
33. Gaveriaux-Ruff C, Kieffer BL (2007) Conditional gene targeting in the mouse nervous system: Insights into brain function and diseases. Pharmacol Ther 113: 619–634. FIND THIS ARTICLE ONLINE
34. Nielsen CS, Price DD, Vassend O, Stubhaug A, Harris JR (2005) Characterizing individual differences in heat-pain sensitivity. Pain 119: 65–74. FIND THIS ARTICLE ONLINE
35. Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, et al. (2006) GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med 12: 1269–1277. FIND THIS ARTICLE ONLINE
36. Taddio A, Goldbach M, Ipp M, Stevens B, Koren G (1995) Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet 345: 291–292. FIND THIS ARTICLE ONLINE
37. Verhoeven K, Timmerman V, Mauko B, Pieber TR, De JP, et al. (2006) Recent advances in hereditary sensory and autonomic neuropathies. Curr Opin Neurol 19: 474–480. FIND THIS ARTICLE ONLINE
38. Wilson SG, Bryant CD, Lariviere WR, Olsen MS, Giles BE, et al. (2003) The heritability of antinociception II: pharmacogenetic mediation of three over-the-counter analgesics in mice. J Pharmacol Exp Ther 305: 755–764. FIND THIS ARTICLE ONLINE
39. Mogil JS, Ritchie J, Smith SB, Strasburg K, Kaplan L, et al. (2005) Melanocortin-1 receptor gene variants affect pain and mu-opioid analgesia in mice and humans. J Med Genet 42: 583–587. FIND THIS ARTICLE ONLINE
40. Smith SB, Marker CL, Perry C, Liao G, Sotocinal SG, et al. (2008) Quantitative trait locus and computational mapping identifies Kcnj9 (GIRK3) as a candidate gene affecting analgesia from multiple drug classes. Pharmacogenet Genomics 18: 231–241. FIND THIS ARTICLE ONLINE
41. Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB (2007) Heritability of responses to painful stimuli in women: a classical twin study. Brain 130: 3041–3049. FIND THIS ARTICLE ONLINE
42. Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, et al. (2007) Individual differences in pain sensitivity: Genetic and environmental contributions. Pain 136: 21–29. FIND THIS ARTICLE ONLINE
43. Klement W, Arndt JO (1992) The role of nociceptors of cutaneous veins in the mediation of cold pain in man. J Physiol 449: 73–83. FIND THIS ARTICLE ONLINE
44. Kato K, Sullivan PF, Evengard B, Pedersen NL (2006) Importance of genetic influences on chronic widespread pain. Arthritis Rheum 54: 1682–1686. FIND THIS ARTICLE ONLINE
45. Lacroix-Fralish ML, Rutkowski MD, Weinstein JN, Mogil JS, Deleo JA (2005) The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine 30: 1821–1827. FIND THIS ARTICLE ONLINE
46. Mogil JS, Ritchie J, Sotocinal SG, Smith SB, Croteau S, et al. (2006) Screening for pain phenotypes: analysis of three congenic mouse strains on a battery of nine nociceptive assays. Pain 126: 24–34. FIND THIS ARTICLE ONLINE
47. Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, et al. (2003) The nature and identification of quantitative trait loci: a community’s view. Nat Rev Genet 4: 911–916. FIND THIS ARTICLE ONLINE
48. Mogil JS, Richards SP, O’Toole LA, Helms ML, Mitchell SR, et al. (1997) Genetic sensitivity to hot-plate nociception in DBA/2J and C57BL/6J inbred mouse strains: possible sex-specific mediation by delta2-opioid receptors. Pain 70: 267–277. FIND THIS ARTICLE ONLINE
49. Wilson SG, Chesler EJ, Hain H, Rankin AJ, Schwarz JZ, et al. (2002) Identification of quantitative trait loci for chemical/inflammatory nociception in mice. Pain 96: 385–391. FIND THIS ARTICLE ONLINE
50. Bergeson SE, Helms ML, O’Toole LA, Jarvis MW, Hain HS, et al. (2001) Quantitative trait loci influencing morphine antinociception in four mapping populations. Mamm Genome 12: 546–553. FIND THIS ARTICLE ONLINE
51. Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, et al. (2007) A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315: 525–528. FIND THIS ARTICLE ONLINE
52. Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, et al. (2005) The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain 6: 159–167. FIND THIS ARTICLE ONLINE
53. Lotsch J, Geisslinger G (2006) Relevance of frequent mu-opioid receptor polymorphisms for opioid activity in healthy volunteers. Pharmacogenomics J 6: 200–210. FIND THIS ARTICLE ONLINE
54. Janicki PK, Schuler G, Francis D, Bohr A, Gordin V, et al. (2006) A genetic association study of the functional A118G polymorphism of the human mu-opioid receptor gene in patients with acute and chronic pain. Anesth Analg 103: 1011–1017. FIND THIS ARTICLE ONLINE
55. Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W (2005) Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem 280: 32618–32624. FIND THIS ARTICLE ONLINE
56. Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, et al. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282. FIND THIS ARTICLE ONLINE
57. Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, et al. (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289. FIND THIS ARTICLE ONLINE
58. Kim H, Mittal DP, Iadarola MJ, Dionne RA (2006) Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet 43: e40. FIND THIS ARTICLE ONLINE
59. Stamer UM, Stuber F (2007) Codeine and tramadol analgesic efficacy and respiratory effects are influenced by CYP2D6 genotype. Anaesthesia 62: 1294–1295. FIND THIS ARTICLE ONLINE
60. Gan SH, Ismail R, Wan Adnan WA, Zulmi W (2007) Impact of CYP2D6 genetic polymorphism on tramadol pharmacokinetics and pharmacodynamics. Mol Diagn Ther 11: 171–181. FIND THIS ARTICLE ONLINE
61. Campa D, Gioia A, Tomei A, Poli P, Barale R (2007) Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther 83: 559–566. FIND THIS ARTICLE ONLINE
62. Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413: 203–210. FIND THIS ARTICLE ONLINE
63. Suzuki M, Mizuno A, Kodaira K, Imai M (2003) Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278: 22664–22668. FIND THIS ARTICLE ONLINE
64. Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ (2004) TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem 279: 21569–21575. FIND THIS ARTICLE ONLINE
65. Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, et al. (2003) COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 299: 1240–1243. FIND THIS ARTICLE ONLINE
66. Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, et al. (2006) Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain 125: 216–224. FIND THIS ARTICLE ONLINE
67. Kim H, Neubert JK, San MA, Xu K, Krishnaraju RK, et al. (2004) Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 109: 488–496. FIND THIS ARTICLE ONLINE
68. Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, et al. (2003) The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A 100: 4867–4872. FIND THIS ARTICLE ONLINE
69. Park JJ, Lee J, Kim MA, Back SK, Hong SK, et al. (2007) Induction of total insensitivity to capsaicin and hypersensitivity to garlic extract in human by decreased expression of TRPV1. Neurosci Lett 411: 87–91. FIND THIS ARTICLE ONLINE
70. Bejaoui K, Wu C, Scheffler MD, Haan G, Ashby P, et al. (2001) SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet 27: 261–262. FIND THIS ARTICLE ONLINE
71. Lafreniere RG, MacDonald ML, Dube MP, MacFarlane J, O’Driscoll M, et al. (2004) Identification of a novel gene (HSN2) causing hereditary sensory and autonomic neuropathy type II through the Study of Canadian Genetic Isolates. Am J Hum Genet 74: 1064–1073. FIND THIS ARTICLE ONLINE
72. Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, et al. (2001) Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet 68: 598–605. FIND THIS ARTICLE ONLINE
73. Lee MJ, Stephenson DA, Groves MJ, Sweeney MG, Davis MB, et al. (2003) Hereditary sensory neuropathy is caused by a mutation in the delta subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct4 ) gene. Hum Mol Genet 12: 1917–1925. FIND THIS ARTICLE ONLINE
74. Lumpkin EA, Caterina MJ (2007) Mechanisms of sensory transduction in the skin. Nature 445: 858–865. FIND THIS ARTICLE ONLINE
75. Foulkes T, Wood JN (2007) Mechanisms of cold pain. Channels 1: 154–160. FIND THIS ARTICLE ONLINE
76. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, et al. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824. FIND THIS ARTICLE ONLINE
77. Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, et al. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313. FIND THIS ARTICLE ONLINE
78. Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, et al. (2004) Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 24: 6410–6415. FIND THIS ARTICLE ONLINE
79. Drew LJ, Rugiero F, Cesare P, Gale JE, Abrahamsen B, et al. (2007) High-threshold mechanosensitive ion channels blocked by a novel conopeptide mediate pressure-evoked pain. PLoS ONE 2: e515. FIND THIS ARTICLE ONLINE
80. Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, et al. (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083. FIND THIS ARTICLE ONLINE
81. Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, et al. (2006) Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 314: 1930–1933. FIND THIS ARTICLE ONLINE
82. Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, et al. (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114: 386–396. FIND THIS ARTICLE ONLINE
83. Trang T, Beggs S, Salter MW (2006) Purinoceptors in microglia and neuropathic pain. Pflugers Arch 452: 645–652. FIND THIS ARTICLE ONLINE
84. Hucho T, Levine JD (2007) Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55: 365–376. FIND THIS ARTICLE ONLINE
85. Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, et al. (2007) Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132: (Supplement 1)S26–S45. FIND THIS ARTICLE ONLINE
86. Craft RM, Mogil JS, Aloisi AM (2004) Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain 8: 397–411. FIND THIS ARTICLE ONLINE
87. Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF (2000) Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev 24: 375–389. FIND THIS ARTICLE ONLINE
88. Fillingim RB, Ness TJ (2000) Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev 24: 485–501. FIND THIS ARTICLE ONLINE
89. Kest B, Palmese C, Hopkins E (2000) A comparison of morphine analgesic tolerance in male and female mice. Brain Res 879: 17–22. FIND THIS ARTICLE ONLINE
90. Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (Dec 2000). “OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity”. Nat Cell Biol 2 (10): 695–702. doi:10.1038/35036318. PMID 11025659
91. Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (Nov 2000). “Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor”. Cell 103 (3): 525–35. PMID 11081638
92. Clapham DE, Julius D, Montell C, Schultz G (Dec 2005). “International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels”. Pharmacol Rev 57 (4): 427–50. doi:10.1124/pr.57.4.6. PMID 16382100. ^ Harteneck C, Plant TD, Schultz G (April 2000). “From worm to man: three subfamilies of TRP channels”. Trends Neurosci. 23 (4): 159–66. doi:10.1016/S0166-2236(99)01532-5. PMID 10717675